Get Tech Tips
Subscribe to free tech tips.
Boyle’s Law

“Robert Boyle” by Stifts- och landsbiblioteket i Skara is licensed under CC BY 2.0
Robert Boyle was an Anglo-Irish philosopher and scientist born in 1627. He had a privileged upbringing, as he was a son of the first earl of Cork, and his social standing gave him access to a first-class education at Eton College. He studied abroad with his brother as a teenager, traveling all over Europe and learning from his experiences. When the Irish rebellion broke out in the early 1640s, Boyle stayed in Vienna to continue his studies away from the political turmoil.
Instead of returning to Ireland, he started his career in England. There, Boyle wrote about ethics and authored some devotional works informed by his Christian faith.
Boyle eventually returned to Ireland and began studying natural philosophy in the 1650s. He joined a few other notable physicists and philosophers and formed the “Experimental Philosophy Club.” Shortly after that, he partnered with Robert Hooke to study pneumatics in 1659, and most of his contributions to society came from his studies of air.
Boyle’s most profound discovery was derived from a report published in 1662. Boyle measured the volume of constant quantities of air compressed by different mercury weights, and he discovered an inverse relationship between the pressure and volume of a gas. This relationship later became known as Boyle’s law.
The law
Boyle’s law states that the volume of a gas varies inversely with the absolute pressure, provided the temperature stays the same. In simpler terms, if you increase the pressure, the volume should decrease. The opposite is also true.
You can use the following equation to represent Boyle’s law:
P1 × V1 = P2 × V2
In short, the product of the original absolute pressure and volume equals the product of the new pressure and volume. This relationship indicates that a pressure increase will cause the volume to decrease and vice versa.
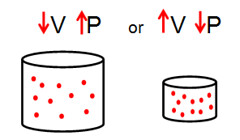
“Boyle's Law, Volume, and Pressure Inverse Relationship” by myers_pictures is licensed under CC BY-NC-SA 2.0
Let’s say you have an initial absolute pressure of 50 PSIA and an absolute volume of 64 in3. The new pressure is lower: 32 PSIA. You can find the new volume by multiplying 50 PSIA by 64 in3 (3200) and then dividing that product by 32.
The volume would be 100 in3, which is higher than the initial absolute volume (64 in3). Even though the new pressure is lower than the original pressure, the new volume is higher than the initial volume. That’s the core of Boyle’s law.
When might we use Boyle’s law in HVAC/R?
When we think about pressure and volume changes, our minds may jump to the compressor. After all, the compressor’s primary function is to apply pressure to reduce the refrigerant’s volume.
However, the gaseous refrigerant will not obey Boyle’s law alone in the compressor because the temperature doesn’t remain constant. The broad idea of Boyle’s law remains true (volume decreases as pressure increases), but the temperature change throws off the exact proportionality.
As you can see, Boyle’s law is impractical for compression as we know it because it requires a constant temperature. However, Boyle’s law works with Charles’s law (or Gay-Lussac’s law) to describe a typical HVAC/R compressor’s function.
Charles’s law establishes an ideal gas’s absolute temperature proportionality to its volume under constant pressure. Charles’s law gives Boyle’s law a more practical basis because it defines temperature’s relationship with the other variables: volume and pressure. They can combine to form the general gas law or law of a perfect gas.
So, although we don’t see Boyle’s law alone in HVAC/R, we see it at work with Charles’s law in a greater equation: the law of perfect gas or ideal gas law. Boyle’s law is a mere component that establishes the proportional relationships between volume, pressure, and temperature.
References
https://www.britannica.com/biography/Robert-Boyle
Special thanks
Modern Refrigeration and Air Conditioning, 21st edition (Andrew Althouse, Carl Turnquist, Alfred Bracciano, Daniel Bracciano, and Gloria Bracciano)
Refrigeration & Air Conditioning Technology, 9th edition (Eugene Silberstein, Jason Obrzut, John Tomczyk, Bill Whitman, and Bill Johnson)
These truly are the reference guides for the industry and deserve attribution for all such articles.









Comments
To leave a comment, you need to log in.
Log In