Get Tech Tips
Subscribe to free tech tips.
Oxidizers and What It Has to Do With COVID-19

Oxidation is the loss of electrons, reduction is the gain of electrons, and the reaction is what occurs between the two. When we talk about chemistry or chemical reactions, we are really talking about an electrical interaction between molecules seeking equilibrium.
In other the words, the old “high voltage goes to low voltage” rule applies to the tiny building blocks that make stuff what it is.
Before we get to viruses, bacteria, and specifically coronavirus COVID-19, let's think about some oxidizing reactions we are more familiar with.
Let's start simple. Let's talk about iron and rust because we see that reaction all the time.
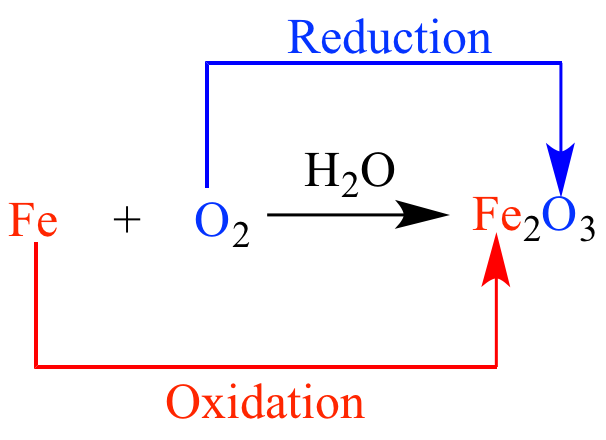
Iron reacts with oxygen in the air and in water (H2O) to “oxidize” and give up electrons, forming a new molecule we call rust. We call this “corrosion” from Latin corrodere, which literally means “to gnaw through,” because that's what it looks like to us.
For the average HVACR tech who is NOT a chemist, this isn't something we are going to opine over on a chalkboard. However, a basic understanding of how and why it occurs can be useful because this corrosion is really a chemical reaction that is, at its heart, an electrical reaction.

Because corrosion (oxidation) is largely undesirable in solids, we work to prevent it by limiting these oxidizing reactions. We even call metals that are less likely to react and oxidize “more noble” and those more likely to react “less noble,” as the chart above shows.
Interestingly, when less noble (anodic) solids are in contact with more noble (cathodic) solids, the less noble will oxidize sacrificially, protecting the more noble metals, like the sacrificial anode shown here.

So, the message is that moving electrons around between those with more than they want and those with fewer than they want causes reactions that can give us some undesirable results. But what does that have to do with coronaviruses?
Oxidization and Air
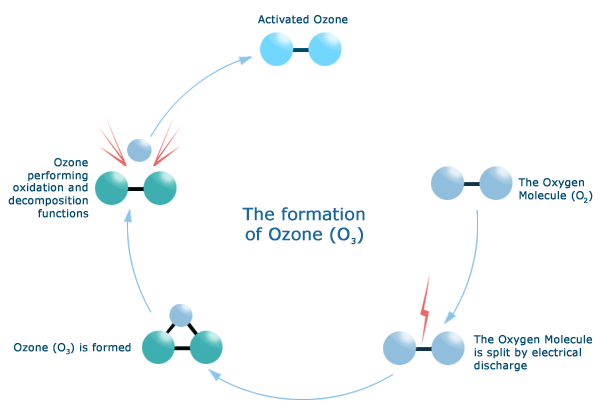
Image taken from an ozone generator marketing piece
We hear about ozone (O3) all the time when we start in the trade and study for our EPA exam. Those instances are all regarding ozone in the upper stratosphere far above the earth, where it protects the earth from harmful UV light.
Ozone also occurs in the lower atmosphere during lightning storms when the electrical energy causes the stable O2 molecule to be broken down to extremely unstable O (only one oxygen atom), combining with other O2 to form slightly less unstable but still VERY unstable O3.
This O3 “reacts” with other molecules readily, resulting in new types of molecules. That is why ozone has been used for some time to reduce odors—the highly reactive O3 combines with other molecules by oxidizing and reducing all over until the reactions stabilize.
Sounds good, right?
Well, the problem is that O3 also reacts with cells in our respiratory system, causing irritation and damage when we breathe it in. Here is a quote from the EPA:
“The same chemical properties that allow high concentrations of ozone to react with organic material outside the body give it the ability to react with similar organic material that makes up the body, and potentially cause harmful health consequences. When inhaled, ozone can damage the lungs. Relatively low amounts can cause chest pain, coughing, shortness of breath and throat irritation. Ozone may also worsen chronic respiratory diseases such as asthma and compromise the ability of the body to fight respiratory infections. People vary widely in their susceptibility to ozone. Healthy people, as well as those with respiratory difficulty, can experience breathing problems when exposed to ozone.”
https://www.epa.gov/indoor-air-quality-iaq/ozone-generators-are-sold-air-cleaners#harmful-ozone
The point is that the oxidizing properties of ozone that make it good at breaking down odors also make it good at breaking down our respiratory system.
On the subject of COVID-19, there is renewed interest in ozone because its oxidizing properties that reduce odors and damage our lungs also make it good at killing viruses, as this poorly translated article points out.
Many home air purifiers use varying amounts of ozone in their processes, some of whom are now using activated carbon to absorb the ozone before it leaves the purification zone, which is a good idea.
Most manufacturers of “active” air purifiers that use oxidization as a strategy no longer seek to use ozone but instead use other ion-based oxidizers, such as:
- Cold Plasma Bi-Polar
- Hydroxyl Radicals
- Ionized Hydrogen Peroxide
The fact remains that all of these ions rely on oxidization as the basis for their operation. There are at least some questions about their safety or effectiveness at the scale of whole-home air purification without careful testing and measurement can be quite difficult to do.
This academic article titled “Illuminating the dark side of indoor oxidants” is a really thoughtful look at what we do and don't know about using oxidants indoors.
When manufacturers of indoor air purification products are questioned, they often isolate the safety and efficacy of their particular oxidizing ion rather than address the challenges related to indoor oxidization as a whole.
This video produced by my friend Corbett Lunsford discusses Hydroxyl-Radicals specifically, which is the type of oxidant produced by PCO technology:
This video covers the unintended consequences that can occur when “incomplete degradation” occurs in oxidization and reduction instead of the reaction that occurs with our lungs and respiratory system, like with O3.
The purpose of this article isn't to throw shade on any particular technology but rather to help illustrate the science behind what we are dealing with when we attempt to neutralize viruses using oxidization.
WARNING – WHAT COMES NEXT IS (LARGELY) MY OPINION, NOT STATEMENTS OF FACT
An example that guides my thinking on this subject is chlorine bleach. It's great at killing viruses and is a very active oxidizer. It plays a role in our everyday life in sanitizing surfaces, and we don't need to be afraid of it… BUT WE DON'T BREATHE IT IN ON PURPOSE.
When we use bleach, we do unintentionally breathe some of the fumes. They are bad for us—all the way bad—but we still have an acceptable tolerance because of the good it does in sanitizing surfaces.
This is how I currently think about airborne oxidizers and why I see that they may have some utility in high-risk microbial environments or where the reactions can be contained and measured.
In the meantime, wash your hands, keep social distance, sanitize work areas, and use good filtration (HEPA if you can) and well-filtered ventilation air as the best bet. (Learn more about HEPA filtration and its effectiveness HERE.)
—Bryan









Comments
Thank you for this information. I have sent this link to the entire staff at my company to further educate themselves. Keep up the good work.
Thank you for this information. I have sent this link to the entire staff at my company to further educate themselves. Keep up the good work.
Not sure where you find the time to publish these articles and/or your podcasts, but thank you for everything you do for this industry.
Not sure where you find the time to publish these articles and/or your podcasts, but thank you for everything you do for this industry.
To leave a comment, you need to log in.
Log In